News
TiNivo-2 Trial Results
Update from ESMO 2024 Data from the TiNivo-2 trial was presented on September 13, 2024, at the European Society for Medical Oncology (ESMO) conference in Barcelona. Below is a recap and discussion of the data that was presented. The thoughts and views that I am sharing about this trial are my own personal opinions. I am not a doctor and this is not medical advice. Background: Most patients diagnosed with metastatic RCC will initially get treatment with one of the following combinations. A major question for doctors and patients is what to do after someone progresses on one of these combination treatments. The TiNivo-2 trial was designed to help answer this question. Trial Design Patients with metastatic RCC who had progressed on treatment with immunotherapy were enrolled in the t...
KCCure Chromophobe RCC Research Grant awarded to Dr. Lisa Henske
The Kidney Cancer Research Alliance (KCCure) announced today that Brigham and Women’s researcher, Dr. Lisa Henske has been selected to receive the KCCure Chromophobe Research Grant Award of $50,000 for her proposal: Targeting KIT in Chromophobe Kidney Cancer. This is the third annual KCCure research grant awarded for Chromophobe Renal Cell Carcinoma (ChRCC), and the second grant to be awarded to Dr. Henske. ChRCC is a rare subtype of kidney cancer accounting for roughly 5 percent of RCC tumors. When discovered early, this histological type is less likely to spread to other parts of the body. However, once the disease becomes metastatic, survival rates are poor. Despite significant advances for patients with the most common type of kidney cancer, research has lagged for pati...
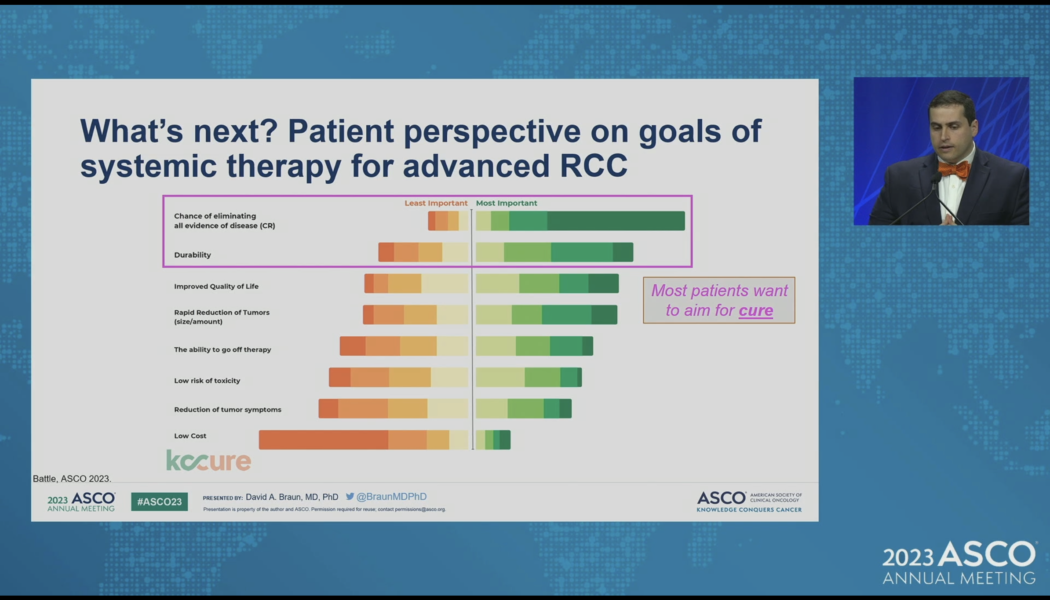
Aim for a Cure – ASCO2023
What do kidney cancer patients want from their therapy? They want a cure. Let’s Aim for a Cure. The big news for kidney cancer doctors at ASCO 2023 may have involved updates from combination trials including CONTACT-03, KEYNOTE426, and CLEAR. For patients, I would say that the big news was this call to action from Dr. David Braun at Yale Cancer Center. Aim for a Cure! Braun did a masterful job of highlighting just how far we have come in kidney cancer – starting with the cytokine era in the 1980s to molecularly targeted agents in the early 2000’s, the first approval of a checkpoint inhibitor in 2015 to the era of combination therapy today. Since the approval of sunitinib in 2006 – the median overall survival for kidney cancer has more than doubled. That is news to celebrate. But patients s...
Chromophobe Research Grant Awarded to Researchers at Brigham and Women’s Hospital
The Kidney Cancer Research Alliance (KCCure) announced today that Dr. Elizabeth Henske and Dr. Carmen Priolo have been selected to receive the FY2022 KCCure Chromophobe Research Grant Award of $50,000 for their proposal: Role of Cysteine Homeostasis and Ferroptosis in the Therapy of Chromophobe RCC. “We are absolutely thrilled to receive this wonderful support from KCCure,” said Drs. Henske and Priolo. “Our work will test the hypothesis that Chomophobe kidney cancer cells are hypersensitive to ferroptosis (a form of iron-dependent cell death). This partnership with KCCure allows us to fast-track critical research, toward highly effective treatments and hopefully a cure for Chromophobe kidney cancer.” Chromophobe Renal Cell Carcinoma (ChRCC) is a rare subtype of kidney ...
Conquering the Data Void
A data void exists when there is a high level of demand for information but little information exists. If you or a loved one has been diagnosed with a rare form of kidney cancer – you have entered a “data void.” Two years ago, you had surgery for a large renal mass. When the pathology came back, “chromophobe renal cell carcinoma,” your doctor told you that you were lucky. Chromophobe renal cell carcinoma (ChRCC) is less aggressive than the most common type, clear cell RCC. It’s rare for ChRCC to recur after surgery. Ironically, the fact that ChRCC is more indolent reduces the sense of urgency when it comes to research and clinical care. So when you found out that your disease has recurred – you entered a data void. There are seventeen FDA approved ...
Scanxiety!
When you wake up this morning, a calendar reminder pops up on your phone. You have an immediate sinking feeling. It’s two weeks until your scan. This is the first scan since you started adjuvant treatment. You feel good and haven’t had too many side effects. But in a weird way, that makes you wonder if the drug is even working. Some moments you feel confident and convinced that this scan will be clear. Other moments, the doubt creeps in and you start imagining what will happen if the scans show recurrent cancer. You try to do some breathing exercises that the counselor gave you, but they aren’t working very well. Two weeks feels like an eternity. One week. You want it to go faster – and go slower at the same time. You dread getting the results of your husband’s next scan – but the un...
Adjuvant Treatment – No Clear Choice
Stage 1 turns out to be Stage 3 You are home recovering from surgery when your pathology report arrives in your medical portal. What your doctor thought was a small, stage 1 renal cell carcinoma was actually a stage 3, grade 2, renal cell carcinoma. Even though the mass was small – less than 5 cm – it invaded the segmented veins – which means it is now stage 3. You don’t fully understand what it all means – but it seems like a pretty big difference from where things stood prior to surgery, and you are feeling blindsided. Having stage 3 disease means that you are eligible for “adjuvant therapy.” Adjuvant therapy is systemic treatment that is given to help prevent or reduce your risk of recurrence. Adjuvant Treatment Decision Making During your follow-up appointme...
You Are Not Alone
Since your kidney cancer diagnosis, you have been struggling emotionally. Your friends and family – the people you rely on the most – have responded in ways that you didn’t expect. Their comments are well intended – but they often come across as hollow and toxically positive. When someone says, “be strong!” – it makes you wonder if they think you aren’t being strong. The words “be positive” feel the same way. Feeling anxious about your health and your future isn’t the same as “being negative.” No one understands how you feel and that makes you feel even more alone. But today – everything changed. Today, you joined an on-line support group for kidney cancer patients. Initially, you just read through the other comments. But then you finally got up the...
COSMIC 313 Clinical Trial
UPDATE FROM ESMO Data from the COSMIC 313 trial was presented on September 12, 2022, at the ESMO conference in Paris. Below is a recap and discussion of the data that was presented. The thoughts and views that I am sharing about this trial are my own personal opinions. I am not a doctor and this is not medical advice. About the Trial COSMIC 313 is a phase 3 clinical trial for kidney cancer patients who have not had any previous treatment for metastatic disease (first-line therapy). The trial tested the triplet of Cabometyx (cabozantinib) + Opdivo (nivolumab) + Yervoy (ipilimumab) to see if using these three drugs in combination works better than the already FDA approved combination of Opdivo (nivolumab) + Yervoy (ipilimumab). The trial is measuring efficacy with two primary endpoints:...