News
Addressing Diagnostic Uncertainty in Localized Kidney Cancer
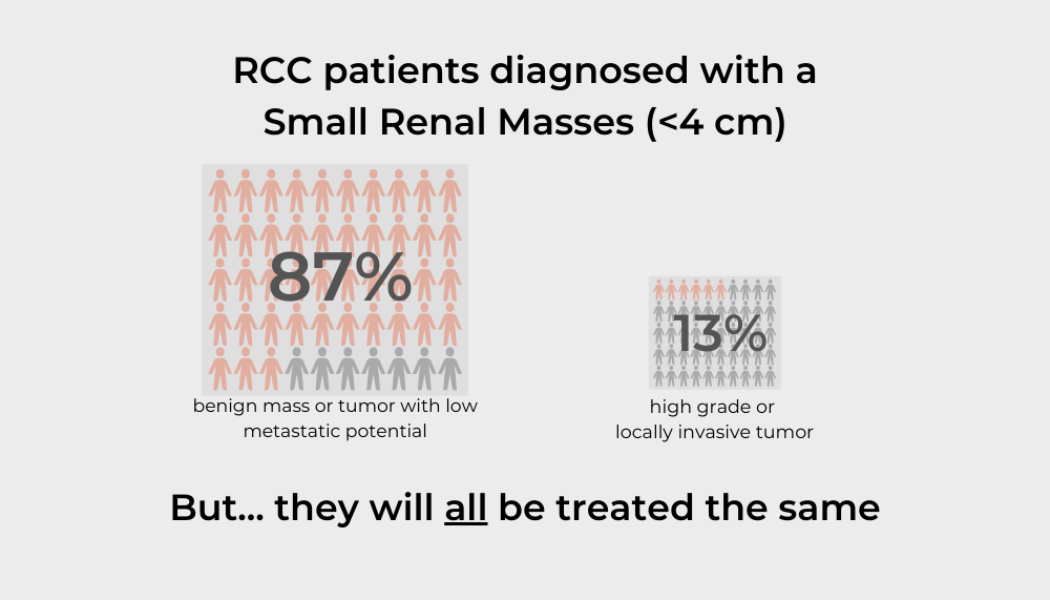
Many patients will be told that they have localized kidney cancer based solely on imaging alone. This can give the impression that diagnostic accuracy of scans is higher than it actually is. While CT scans are predictive of renal cell carcinoma and are routinely used to recommend surgery – the only definitive way to know for sure whether a renal mass is cancer is surgical removal. As many as 20 percent of patients who undergo surgery for suspected RCC will not have kidney cancer. Expanded access to CT scans has resulted in increased detection of small masses (under 4 cm) confined to the kidney. Nearly half of kidney cancer diagnoses today are stage 1 renal tumors. Research shows that at least 20 percent of these masses will be benign. Eighty percent of small renal masses that are malignan...
Voice of Kidney Cancer – The Story of Benny
Sometimes Angels Don’t Have Wings – They Have Paws Eleven years ago I was diagnosed with renal cell carcinoma. If you or a loved one has been impacted by cancer, I don’t have to tell you what that day was like. Almost to the day, Benny came into my life. He was small and all he wanted to do was be with me all of the time. Since my initial diagnosis, I have endured five surgeries, had thousands of doctors appointments and spent time in and out of the hospital. Through it all, little Benny was always there for me. All he asked in return was an occasional belly rub and to be able to sleep beside me. Last October, doctors told me the amazing news that my scans were clear and there was no detectable cancer in my body. But as my health improved, Benny’s health declined. H...
Rare Disease Day 2022
In the United States, a rare disease is defined as a disease that impacts fewer than 200,000 people. In the European Union, it is defined as a disease that effects 1 in 2,000. Kidney cancer patients know they have a rare disease because they hear this: “We hardly ever see this subtype of kidney cancer…” “There are treatments we can try, but we don’t know if they will work for you.” Most patients diagnosed with kidney cancer will have clear cell renal cell carcinoma. But around 30 percent of patients will be diagnosed with a subtype other than clear cell, such as papillary RCC, chromophobe RCC, translocation RCC or unclassified RCC (a mix of multiple subtypes). The list of newly identified subtypes continues to expand as science evolves. Patients with rare subtypes have higher a...
Kidney Cancer Awareness – March 2022
March is Kidney Cancer Awareness Month! Over 400,000 people worldwide will be diagnosed with kidney cancer this year. In the United States, 500,000 Americans are living with kidney cancer and 70,000 more will be diagnosed. March is our month to raise awareness about the impact of this disease and the need for more research. Over the last few months, the world has been captivated by shared puzzles. We can’t imagine a better puzzle to solve than finding CURES for kidney cancer patients. Be part of the solution this March! LEARN – SHARE – FUND LEARN: One of the biggest impediments to advancing cures for kidney cancer is a lack of understanding and awareness. Get the facts! Throughout the month – we will be posting Challenge Questions on our Facebook Page – ...
KCCure Announces New Research Grant For Chromophobe Renal Cell Carcinoma
November 19, 2021 KCCure is offering a grant totaling $50,000 for a project focused on innovative scientific research in chromophobe renal cell carcinoma (RCC). Chromophobe RCC is a rare subtype of kidney cancer accounting for roughly 5 percent of RCC tumors. When discovered early, this histological type is less likely to spread to other parts of the body. However, once the disease becomes metastatic, survival rates are poor. Over the past decade, more than seventeen new treatments have been approved by the FDA to treat kidney cancer. However, all of these treatments have been established in clear cell RCC patients, the most common form of the disease. No treatments have been identified for chromophobe RCC. According to Dr. Kimryn Rathmell, chair of medicine at Vanderbilt Unive...
The Unanswered Question
The FDA approval of adjuvant Keytruda is a significant advancement in the treatment of renal cell carcinoma. Immune based adjuvant therapy provides an exciting new option for a patient population in desperate need of improved outcomes. Over the past four years, KCCure has strived to be a voice for patients diagnosed with localized disease. Our surveys uncovered profoundly high levels of fear of cancer recurrence (FCR) in this patient population. Additional data revealed that high anxiety levels were independent of stage (and risk). We presented data at multiple medical conferences showing that patients want adjuvant therapy and that they are willing to use it regardless of overall survival benefit or toxicity levels. We can celebrate the achievement that this approval represents – w...
FDA approves the combination of lenvatinib (Lenvima) plus pembrolizumab (Keytruda) for first-line treatment of advanced renal cell carcinoma (RCC)
On August 10, 2021, the Food and Drug Administration approved the combination of lenvatinib (Lenvima) plus pembrolizumab (Keytruda) for first-line treatment of adult patients with advanced renal cell carcinoma (RCC). Pembrolizumab is an anti-PD-1 checkpoint inhibitor that helps the immune system detect and fight tumor cells. Lenvatinib is an anti-angiogenic targeted therapy that inhibits growth of tumors. This is the fifth combination treatment to be approved for the treatment of advanced kidney cancer. The efficacy of this combination was investigated in CLEAR (Study 307/KEYNOTE-581; NCT02811861), a multicenter, open-label, randomized phase 3 trial in patients with advanced RCC in the first-line setting. Progression-free survival (PFS) and overall survival (OS) were the major efficacy end...
Patient Driven Research – the KCCure Chromophobe Research Grant
“Has anyone done any fundraising or advocacy projects to help raise money or awareness for Chromophobe RCC? Every specialist I talk to mentions the limited research in chromophobe since we are not as common as clear cell.” – Catherine Yutmeyer, chromophobe kidney cancer patient At KCCure, we pride ourselves on being an evidence-based, patient-driven advocacy organization. But when I saw this post in our chromophobe patient community, I realized that we were relying too heavily on providers to set the priorities for our research grant program. To be true to our values, we needed to bring patients into the process. Rare Subtypes Our communities for rare subtypes of kidney cancer have grown dramatically over the past few years. Over and over again, we hear the desperation of people rais...
In Remembrance 2020
This year, more than 150,000 people died worldwide from kidney cancer. This video represents just a fraction of the toll that this disease has taken. Please take a moment to honor and remember those that we lost this year.
Fear of Cancer Recurrence – and why it matters so much
Seventy-five percent of patients will be diagnosed with localized disease – cancer that is confined to the kidney. For most of these patients, surgery will be curative. Because of that fact, few resources exist to support these patients. The assumption is that they are “cured” and no longer need additional care. They are “lucky.” The problem with this logic is that 20 to 40 percent of those patients will eventually face a recurrence in their lifetime. Patients live in fear, wondering if they will be one of those people. While algorithms have been created, there still isn’t any consensus on how to risk stratify patients with localized disease. There is also no consensus on the type or frequency of follow-up care they should receive. Many patients are unaw...