Kidney Cancer clinical trials provide an exciting opportunity to be at the forefront of cancer treatment. In this Making a Difference article, KCCure highlights the work being done by Calithera Biosciences. Laura Loughlin, KCCure’s Director of Patient Engagement, had the opportunity to speak with Catherine Sunderlin, Senior Medical Science Liaison at Calithera Biosciences to discuss their CANTATA kidney cancer clinical trial and their oncology drug candidate telaglenastat (CB-839).
KCCure: Thank you for taking the time to meet with us Catherine. Can you tell us about Calithera Biosciences?
Catherine Sunderlin: Calithera Biosciences is a pharmaceutical company founded in 2010 and based in South San Francisco, CA. Our vision is to be a fully integrated biotechnology company that develops small molecule drugs. We use an onco-metabolism approach to bring a new and unique perspective to fighting cancer. Our goal is to commercialize therapies that make a meaningful difference for patients fighting cancer. The drugs we develop are designed with the intent of targeting unique metabolic pathways, including tumor metabolism, immune cell metabolism and tissue metabolism. Today’s focus is on our glutaminase inhibitor drug candidate telaglenastat (CB-839), which we have demonstrated in pre-clinical studies, targets tumor metabolism.
KCCure: Has CB-839 shown promise in fighting renal cell carcinoma?
Catherine Sunderlin: Yes, we have seen encouraging data in Phase 1 clinical studies! As published in the proceedings from the ASCO Genitourinary Conference, a Phase 1 study of CB-839 combined with cabozantinib showed a 40% overall response rate and 100% disease control rate in patients with clear cell advanced/metastatic RCC. Since this publication, the overall response rate has increased to 50% Calithera Press Release (10/05/18). As noted in the METEOR Phase 3 trial, use of cabozantinib alone had a 17% overall response rate.
Based on the positive Phase 1 results, Calithera has initiated a Phase 2 randomized, double blind, placebo-controlled trial called CANTATA. This study is designed to evaluate the safety and efficacy of CB-839 in combination with cabozantinib compared to cabozantinib with placebo. It is a global trial in recruiting status, with locations in France, UK, Spain, Italy, Germany Australia, New Zealand and 50 US cities. The trial is for patients with metastatic clear cell renal cell carcinoma in 2nd or 3rd line therapy, following treatment with an anti-VEGF or the immunotherapy combination of nivolumab plus ipilimumab.
KCCure: Can you explain how a drug like CB-839 works to fight cancer?
Catherine Sunderlin: CB-839 has a unique proposed mechanism of action.
To support rapid tumor growth there 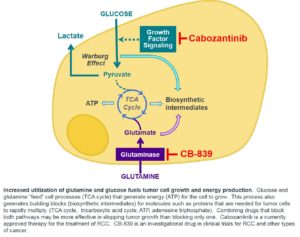 are metabolism changes in tumor cells. Pre-clinical studies show that certain tumor cells need increased amounts of the nutrients glucose and glutamine to grow or survive. For tumor cells to utilize glutamine, the enzyme glutaminase must first convert glutamine to glutamate. CB-839 inhibits glutaminase so this conversion does not happen, and the cells energy source is cut off. Removing the cancer cell’s ability to process glutamine can lead to a major reduction in cell growth, and for some types of cancer, cell death; however, normal cells do not show this pronounced dependence on glutamine. Based on preclinical data, we believe several specific tumor types will be sensitive to glutaminase inhibition and benefit from treatment with CB-839, one being renal cell carcinoma.
are metabolism changes in tumor cells. Pre-clinical studies show that certain tumor cells need increased amounts of the nutrients glucose and glutamine to grow or survive. For tumor cells to utilize glutamine, the enzyme glutaminase must first convert glutamine to glutamate. CB-839 inhibits glutaminase so this conversion does not happen, and the cells energy source is cut off. Removing the cancer cell’s ability to process glutamine can lead to a major reduction in cell growth, and for some types of cancer, cell death; however, normal cells do not show this pronounced dependence on glutamine. Based on preclinical data, we believe several specific tumor types will be sensitive to glutaminase inhibition and benefit from treatment with CB-839, one being renal cell carcinoma.
KCCure: How would a patient take CB-839. Is it tolerated well in general?
Catherine Sunderlin: It is an oral drug, a full dose being four pills, taken twice a day. CB-839 appears to be well tolerated at dosages needed to slow or stop tumors from growing. Side effects reported were manageable with the most common being fatigue, nausea, changes in liver function, and photophobia. As with any clinical trial a participating patient would be monitored closely.
KCCure: Who can participate in the CANTATA clinical trial?
Catherine Sunderlin: Criteria to participate in the clinical trial include, but are not limited to:
- Diagnosis of RCC with clear cell component
- Karnofsky performance status ≥70% (determined by your doctor, this scale measures the ability of a patient to tolerate therapies).
- Measurable disease
- 1 or 2 prior lines systemic therapy including at least 1 anti-angiogenic therapy or combination of nivolumab + ipilimumab
You would not quality if any of the following apply:
- Prior CB-839 or cabozantinib (or another MET inhibitor)
- Untreated brain metastases or central nervous system (CNS) cancer
- Major surgery within 6 weeks or significant bleeding within 3 months
- Past small bowel resection or gastric bypass surgery
Reference the clinical trial here for full details on the inclusion and exclusion criteria.
KCCure: Where can we find more details on your web site?
Catherine Sunderlin: A great one page summary can be found here Other sources of info include:
CANTATA is recruiting now. Please talk with your oncologist if you are interested, and your oncologist can help determine if you meet the criteria to enroll.
KCCure is proud to share the amazing work being done by Calithera Biosciences, and thanks Catherine Sunderlin for taking the time to meet with us.
Kidney Cancer clinical trials provide an exciting opportunity to be at the forefront of cancer treatment. In this Making a Difference article, KCCure highlights the work being done by Calithera Biosciences. Laura Loughlin, KCCure’s Director of Patient Engagement, had the opportunity to speak with Catherine Sunderlin, Senior Medical Science Liaison at Calithera Biosciences to discuss their CANTATA kidney cancer clinical trial and their oncology drug candidate telaglenastat (CB-839).
KCCure: Thank you for taking the time to meet with us Catherine. Can you tell us about Calithera Biosciences?
Catherine Sunderlin: Calithera Biosciences is a pharmaceutical company founded in 2010 and based in South San Francisco, CA. Our vision is to be a fully integrated biotechnology company that develops small molecule drugs. We use an onco-metabolism approach to bring a new and unique perspective to fighting cancer. Our goal is to commercialize therapies that make a meaningful difference for patients fighting cancer. The drugs we develop are designed with the intent of targeting unique metabolic pathways, including tumor metabolism, immune cell metabolism and tissue metabolism. Today’s focus is on our glutaminase inhibitor drug candidate telaglenastat (CB-839), which we have demonstrated in pre-clinical studies, targets tumor metabolism.
KCCure: Has CB-839 shown promise in fighting renal cell carcinoma?
Catherine Sunderlin: Yes, we have seen encouraging data in Phase 1 clinical studies! As published in the proceedings from the ASCO Genitourinary Conference, a Phase 1 study of CB-839 combined with cabozantinib showed a 40% overall response rate and 100% disease control rate in patients with clear cell advanced/metastatic RCC. Since this publication, the overall response rate has increased to 50% Calithera Press Release (10/05/18). As noted in the METEOR Phase 3 trial, use of cabozantinib alone had a 17% overall response rate.
Based on the positive Phase 1 results, Calithera has initiated a Phase 2 randomized, double blind, placebo-controlled trial called CANTATA. This study is designed to evaluate the safety and efficacy of CB-839 in combination with cabozantinib compared to cabozantinib with placebo. It is a global trial in recruiting status, with locations in France, UK, Spain, Italy, Germany Australia, New Zealand and 50 US cities. The trial is for patients with metastatic clear cell renal cell carcinoma in 2nd or 3rd line therapy, following treatment with an anti-VEGF or the immunotherapy combination of nivolumab plus ipilimumab.
KCCure: Can you explain how a drug like CB-839 works to fight cancer?
Catherine Sunderlin: CB-839 has a unique proposed mechanism of action. To support rapid tumor growth there 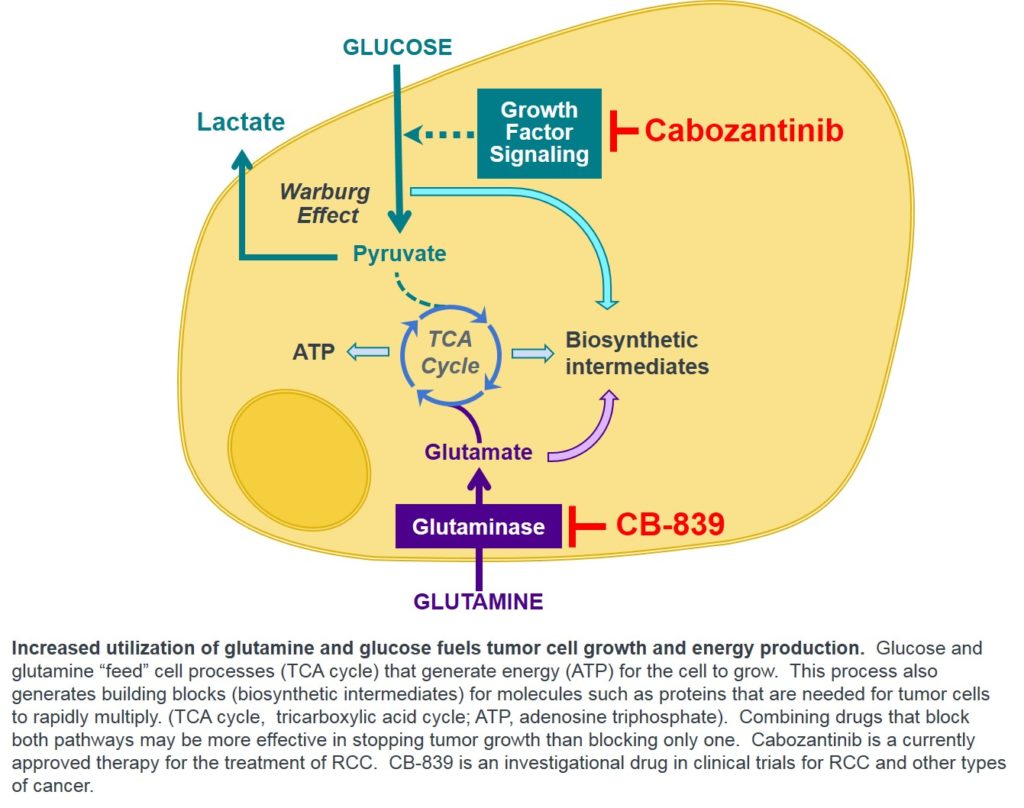 are metabolism changes in tumor cells. Pre-clinical studies show that certain tumor cells need increased amounts of the nutrients glucose and glutamine to grow or survive. For tumor cells to utilize glutamine, the enzyme glutaminase must first convert glutamine to glutamate. CB-839 inhibits glutaminase so this conversion does not happen, and the cells energy source is cut off. Removing the cancer cell’s ability to process glutamine can lead to a major reduction in cell growth, and for some types of cancer, cell death; however, normal cells do not show this pronounced dependence on glutamine. Based on preclinical data, we believe several specific tumor types will be sensitive to glutaminase inhibition and benefit from treatment with CB-839, one being renal cell carcinoma.
are metabolism changes in tumor cells. Pre-clinical studies show that certain tumor cells need increased amounts of the nutrients glucose and glutamine to grow or survive. For tumor cells to utilize glutamine, the enzyme glutaminase must first convert glutamine to glutamate. CB-839 inhibits glutaminase so this conversion does not happen, and the cells energy source is cut off. Removing the cancer cell’s ability to process glutamine can lead to a major reduction in cell growth, and for some types of cancer, cell death; however, normal cells do not show this pronounced dependence on glutamine. Based on preclinical data, we believe several specific tumor types will be sensitive to glutaminase inhibition and benefit from treatment with CB-839, one being renal cell carcinoma.
KCCure: How would a patient take CB-839. Is it tolerated well in general?
Catherine Sunderlin: It is an oral drug, a full dose being four pills, taken twice a day. CB-839 appears to be well tolerated at dosages needed to slow or stop tumors from growing. Side effects reported were manageable with the most common being fatigue, nausea, changes in liver function, and photophobia. As with any clinical trial a participating patient would be monitored closely.
KCCure: Who can participate in the CANTATA clinical trial?
Catherine Sunderlin: Criteria to participate in the clinical trial include, but are not limited to:
- Diagnosis of RCC with clear cell component
- Karnofsky performance status ≥70% (determined by your doctor, this scale measures the ability of a patient to tolerate therapies).
- Measurable disease
- 1 or 2 prior lines systemic therapy including at least 1 anti-angiogenic therapy or combination of nivolumab + ipilimumab
You would not quality if any of the following apply:
- Prior CB-839 or cabozantinib (or another MET inhibitor)
- Untreated brain metastases or central nervous system (CNS) cancer
- Major surgery within 6 weeks or significant bleeding within 3 months
- Past small bowel resection or gastric bypass surgery
Reference the clinical trial here for full details on the inclusion and exclusion criteria.
KCCure: Where can we find more details on your web site?
Catherine Sunderlin: A great one page summary can be found here Other sources of info include:
CANTATA is recruiting now. Please talk with your oncologist if you are interested, and your oncologist can help determine if you meet the criteria to enroll.
KCCure is proud to share the amazing work being done by Calithera Biosciences, and thanks Catherine Sunderlin for taking the time to meet with us.








