UPDATE FROM ESMO
Data from the COSMIC 313 trial was presented on September 12, 2022, at the ESMO conference in Paris. Below is a recap and discussion of the data that was presented. The thoughts and views that I am sharing about this trial are my own personal opinions. I am not a doctor and this is not medical advice.
About the Trial
COSMIC 313 is a phase 3 clinical trial for kidney cancer patients who have not had any previous treatment for metastatic disease (first-line therapy). The trial tested the triplet of Cabometyx (cabozantinib) + Opdivo (nivolumab) + Yervoy (ipilimumab) to see if using these three drugs in combination works better than the already FDA approved combination of Opdivo (nivolumab) + Yervoy (ipilimumab).
The trial is measuring efficacy with two primary endpoints: Progression free survival (PFS) and Overall survival (OS). PFS measures how long the treatments control tumor growth and OS measures how long patients live.
Modest Benefit
In the graphic below – you can see the difference in progression free survival for the two different arms.
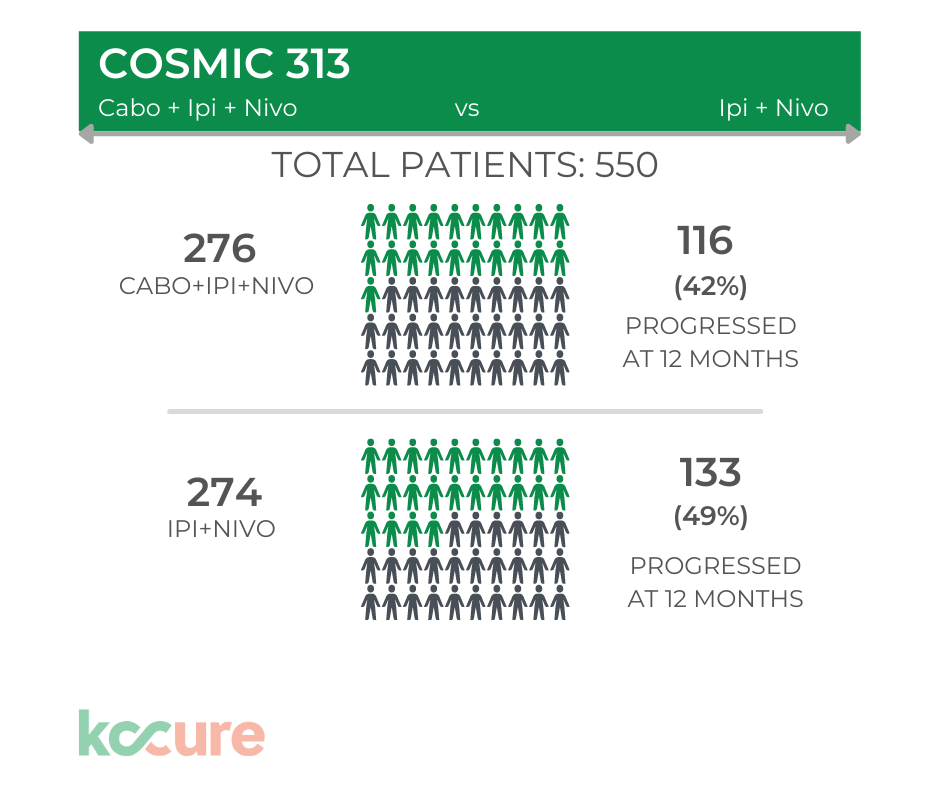
The results show a modest improved rate of progression free survival for the new triplet versus the control arm. After 12 months of therapy, 133 patients had progressed on Ipi/Nivo alone. 113 patients had progressed on Cabo/Ipi/Nivo.
Patients recieving the triplet had higher rates of partial response (reduction of disease by more than 30%) and stable disease, and lower rates of progressive disease. But only 7 patients had a complete response on the triplet compared with 9 patients on Ipi/Nivo alone.
Concerning Toxicity Rates
Conversely, as you can see in the graphic below, the toxicity rates with the triplet were significantly higher than with just Ipi/Nivo alone.
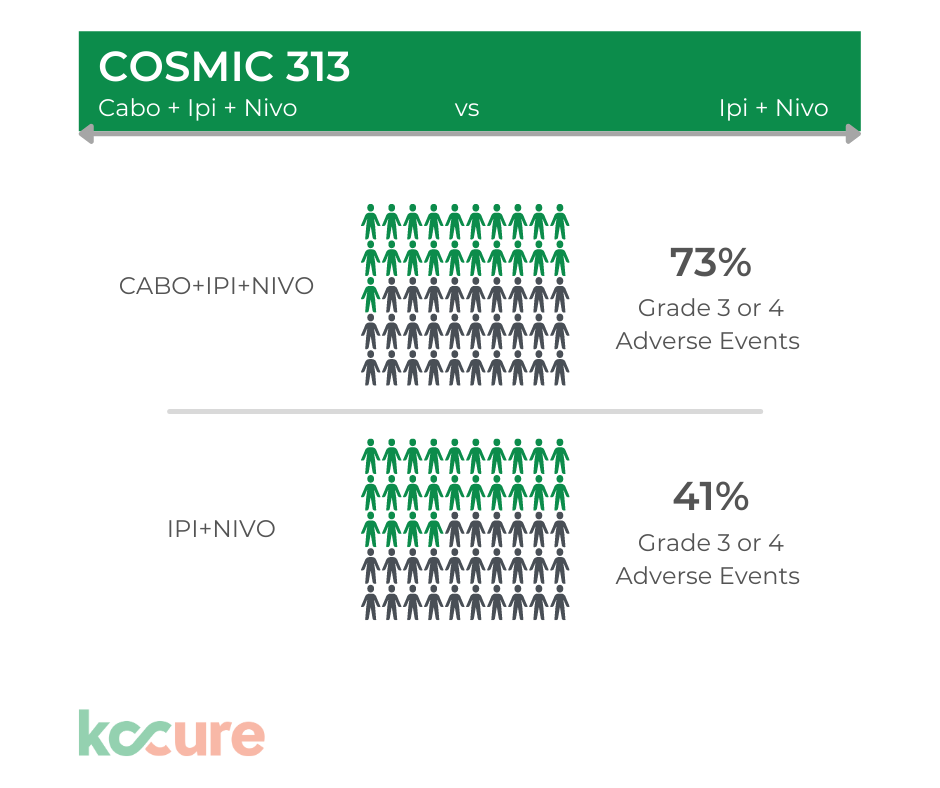
Grade 3 are severe and undesirable adverse events (e.g., significant symptoms requiring hospitalization or invasive intervention; Grade 4 are life threatening or disabling adverse events (e.g., complicated by acute, life- threatening metabolic or cardiovascular complications; need for intensive care or emergent invasive procedure; emergent interventional radiological procedure, therapeutic endoscopy or operation).
Liver Toxicity
The most concerning side effect with the triplet is liver related toxicity. For patients on the triple combination, 26% experienced grade 3 AST elevation and 20% experienced ALT elevation. That’s four times higher than what was seen in the Ipi/Nivo alone arm.
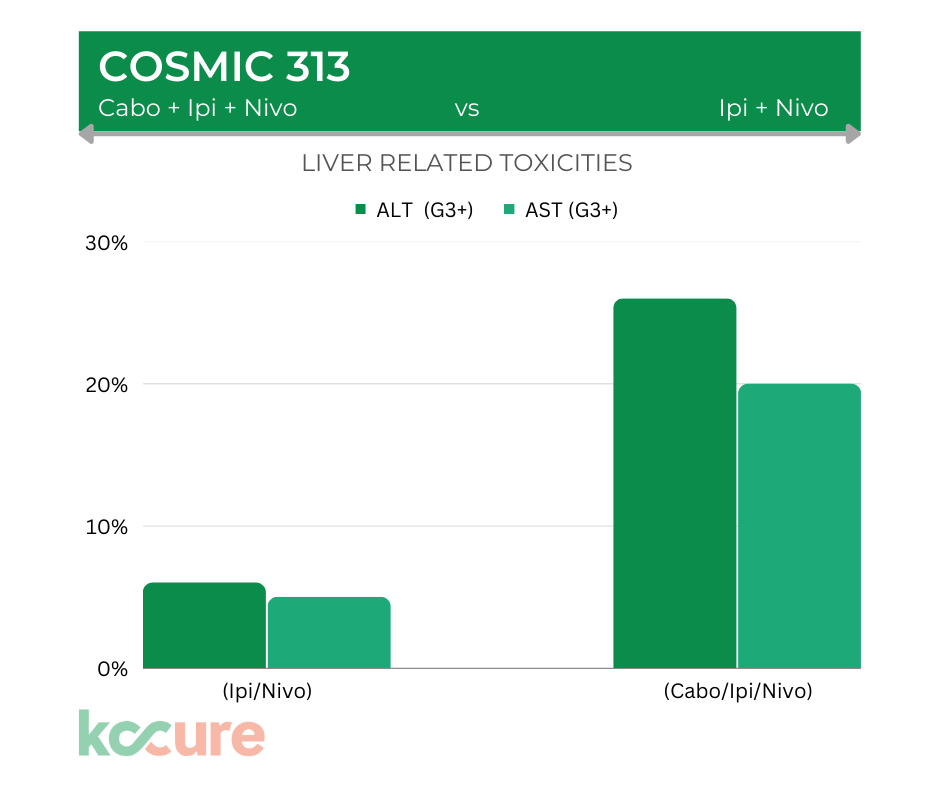
Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are two liver enzymes healthcare providers look at when trying to figure out if you have a problem with your liver.
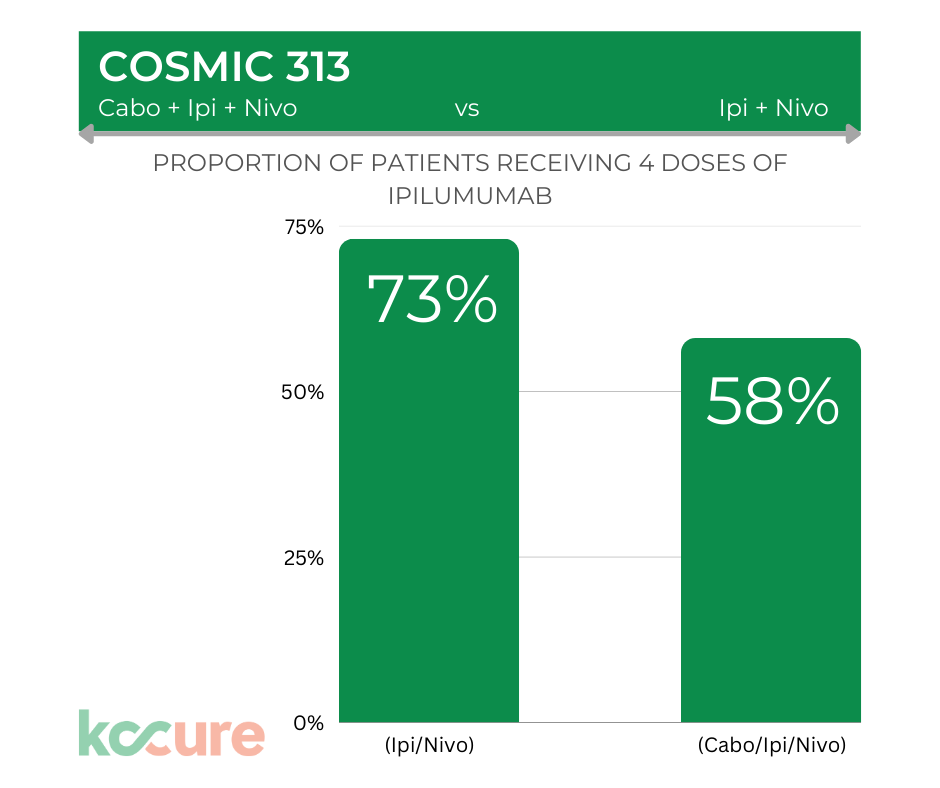
High Rate of Treatment Discontinuation
Patients on the triplet therapy were less likely to be able to get full doses of all three drugs. Only 58% of patients were able to get all four doses of Yervoy (ipilimumab) versus 73% of patients in the control arm. That raises the question about whether patients might have been able to stay on certain treatments longer if the therapies had been sequenced – rather than combined.
What Happens Next?
The trial for COSMIC 313 has completed accrual, so no other patients will be enrolled. While the progression free survival data do show a modest benefit for Cabo/Ipi/Nivo, the sponsors will wait for the overall survival data to be available before making a decision about whether to file for FDA approval.
Without overall survival data, it’s impossible to know whether this triplet therapy really is improving outcomes – or whether the additional toxicity is harming patients in the long run.
My Personal View
I think patients are willing to accept significant toxicities – but they do so in exchange for the chance of achieving a significant benefit. I am not sure that the benefits shown in this trial outweigh the toxicity risks. I am concerned that some patients who are treated with this triplet lose out on the opportunity for a response that could have been achieved with doublet therapy. I am also concerned that liver toxicity could prevent or delay access to second line therapy for patients who progress.
We are all hopeful for new treatments that will attack cancer quickly, resulting in complete and durable responses upfront. However, the results from this trial give me pause. I am not sure this is the triplet we are looking for.








