Update from ESMO 2024
Data from the TiNivo-2 trial was presented on September 13, 2024, at the European Society for Medical Oncology (ESMO) conference in Barcelona. Below is a recap and discussion of the data that was presented. The thoughts and views that I am sharing about this trial are my own personal opinions. I am not a doctor and this is not medical advice.
Background:
Most patients diagnosed with metastatic RCC will initially get treatment with one of the following combinations.

A major question for doctors and patients is what to do after someone progresses on one of these combination treatments.


The TiNivo-2 trial was designed to help answer this question.
Trial Design
Patients with metastatic RCC who had progressed on treatment with immunotherapy were enrolled in the trial.
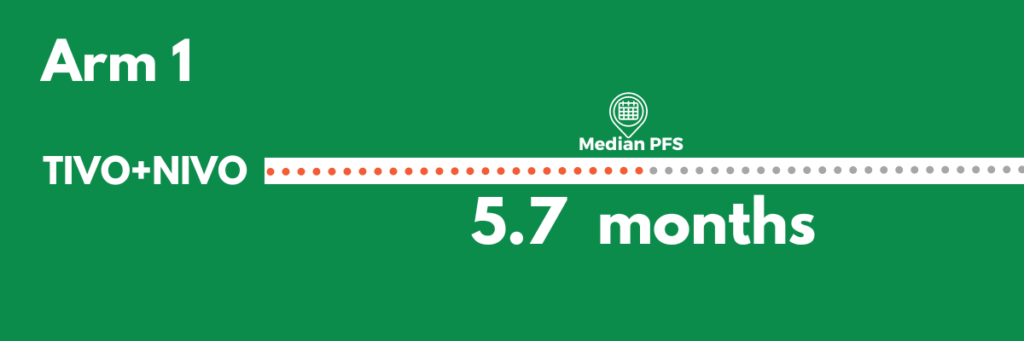
Arm 1: Patients were treated with tivozanib (Fotivda – targeted oral agent) combined with nivolumab (Opdivo – immunotherapy).
Arm 2: Patients were given tivozanib (Fotivda – targeted oral agent) alone.
Results
Patients in the combination treatment arm had a median progression free survival of 5.7 months.
Patients in the control arm, treated with single agent tivozanib, had a median progression free survival of 7.4 months.

Bottom Line: Patients who continued with immunotherapy did worse than patients who received single agent treatment with tivozanib.
The trial also showed that patients who had most recently been treated with Immunotherapy (IO) and had fewer lines of therapy had an even longer progression free survival, indicating that tivozanib (Fotivda) might be a good drug to consider as a second line treatment.

Data from this trial was published in The Lancet with the following conclusion:

What does this mean for patients?
This is the second trial to show that continuing treatment with immunotherapy following progression results in worse outcomes.
The CONTACT03 trial testing combination atezolizumab (immunotherapy)+ cabozantinib compared to cabozantinib alone also found that patients who continued getting immunotherapy had shorter progression free survival compared to patients who were treated with single agent targeted treatment.
The TiNIVO trial in addition to the previous trial will likely result in changes to practice recommendations about continuing immunotherapy following progression.
Does this mean that patients can NEVER get treated with immunotherapy again if you previously progressed?
There is still a great deal of uncertainty about how to sequence treatments and when to rechallenge drugs. No doubt, doctors and patients will continue to experiment with various strategies.
What this trial does help to clarify is that continuing to use immunotherapy after progression should not be standard of care. It also should give comfort to patients that treatment with single agents does not result in reduced outcomes.








