Harnessing the Full Potential of Immunotherapy: Radiation Induced Vaccination and Dual Immune Checkpoint Blockade (RADVAX Kidney Cancer Trial) – kidney cancer immunotherapy treatment.
Objective: To Increase the Efficacy of Potent Immunotherapy In Cancer Patients
Background:
Unleashing the immune system by inhibiting its natural breaks (i.e. immune checkpoints) has started an unprecedented revolution in cancer treatment. It represents arguably one of the most significant advances for the treatment of cancer and is transforming the care for cancer patients.
In particular immune checkpoint inhibitors targeting the PD1/PD-L1, such as nivolumab (NIVO), pathway have shown broad activity across tumor types, including kidney cancer. With monotherapy alone we expect around 20% of patients to experience significant tumor shrinkage and half of those (~10%) of patients durable cancer control.
Taking two breaks of the immune system by adding another immune checkpoint inhibitor, ipilimumab (IPI), leads to a significant improvement in the response rate to around 40% and represents the most potent immunotherapy in kidney cancer to date (NIVO/IPI combination, Hammers et. al. ASCO 2014/15). However, this approach comes at the cost of increased autoimmune related toxicities and probably represents the limit of what can be safely achieved with just taking the breaks of the immune system. Thus, other complementary, potentially synergizing approaches are needed.
Inflamed versus Non-Inflamed Tumors
There is one overriding limitation of immune checkpoint inhibitors: they can only restore an already ongoing immune response in patients. That means the immune system must have recognized the cancer and already started an immune response, which is actively suppressed by the cancer can then be unleashed with NIVO/IPI treatments. Such tumors are typically inflamed and show signs of immune cells infiltration (Figure 1) and upregulation of the PDL1 marker.
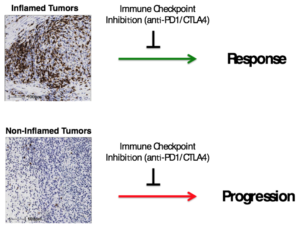
Figure 1:
Inflamed tumors show infiltration of immune cells. (CD8+ T cells, brown staining). These tumors are being recognized by the immune system and are more likely to respond to immune checkpoint inhibition.
Non-inflamed tumors are not being recognized by the immune system and patients are much less likely to benefit from immune checkpoint inhibition. The goal is to cause recognition of these tumors by causing cell death and inflammation.
Radiation-Induced Vaccination (RADVAX).
Our goal is to add the critical prerequisite for an effective immune response and to induce the immune recognition of a patient’s individual tumor. In animal models this can be achieved by combining high doses of radiation (also known as Cyberknife, stereotactic body radiation therapy (SBRT) (Figure 2). In this setting, the addition of potent dual immune check blockade has shown dramatic effects in an aggressive mouse model of cancer (Victor et al, Nature 2015) . Systemic (body wide, abscopal) effects after high dose radiation of kidney cancer have been described in patients (Wersaell et al.), but benefit was limited – probably due to immune checkpoints. Thus radiating 1-2 tumor sites could start the initial immune response (i.e. radiation-induced vaccination), which can then be unleashed with dual immune checkpoint blockade (NIVO/IPI).
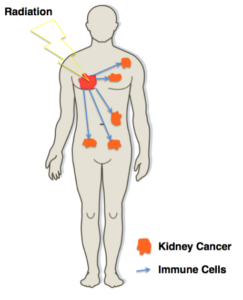
Figure 2:
Radiation and the Abscopal Effect:
High-dose radiation (SBRT) can effectively destroy even kidney cancer cell locally. This will induce a local inflammation at the radiation site and create visibility to the immune. It functions effectively as a personalized vaccine which can start an immune response against the cancer and have effects against other non-radiated tumors. The chances for this to occur are significantly higher when the immune system is maximally unleashed during dual immune checkpoint blockade (NIVO/IPI).
We have evidence for this in animals, the goal is to bring this concept to patients.
Applications beyond Kidney Cancer
We are proposing a proof-of-principle trial, the first of its kind, to combine the most potent immunotherapy to date (dual immune checkpoint blockade with NIVO/IPI) and high-dose radiation induced vaccination. This approach is certainly not limited to kidney cancer, but could become a model for any solid tumor such as lung, breast, prostate and colon cancer for example
Clinical Trial Design:
We have two major goals with our clinical trial:
- To determine the safety and efficacy of combined SBRT and dual immune checkpoint blockade
- To assess blood and tumor tissues for immune response related biomarkers, which will allow us to determine mechanisms of resistance of resistance of response. A thorough understanding would help us in patient selection and further improving immunotherapies.
Study Schema:
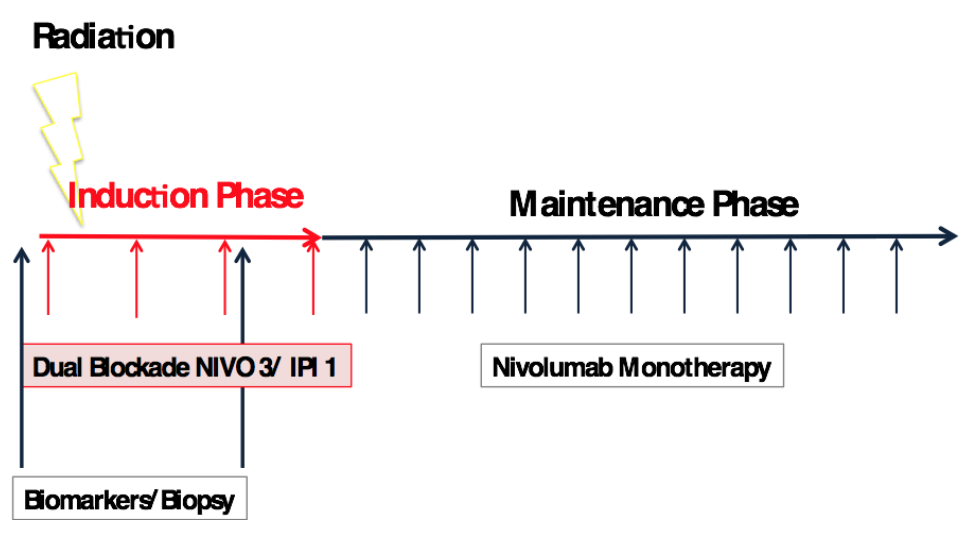
Eligible, treatment-naïve or pretreated patients are started on NIVO/IPI combination therapy and will receive stereotactic, high dose radiation to 1-2 of their tumor sites during the first two weeks of immunotherapy. After up to four doses of the combination (induction phase), patients will go onto nivolumab monotherapy for up to two years.
The statistical design requires 25 patients to determine whether the addition of SBRT to NIVO/IPI is superior to NIVO/IPI alone. The minimum requirement for an initial impression (futility analysis is 15 patients). Tissue biopsies are required at baseline and during week 6-7 of combinations therapy.
Key Personnel:
 Medical Oncology: Hans Hammers MD, PhD
Medical Oncology: Hans Hammers MD, PhD
Dr. Hammers is a recognized leader in the field of immunotherapy and kidney cancer. For example, he has been leading the first NIVO/IPI trial in kidney cancer and has presented the data at international meetings. He also runs a lab which is focused on developing novel therapies in kidney cancer and translational research. He is Co-Leader of Clinical Research and Immunotherapy of the Kidney Cancer Program at UT Southwestern.
 Radiation Oncology: Danny Y Song MD
Radiation Oncology: Danny Y Song MD
Dr. Song is a recognized leader in the field of radiation oncology. Dr. Song’s clinical activities involve treating patients with cancers of the genitourinary tract (kidney, prostate, bladder, testicular cancers) and cancers of the lung, for which he uses external beam, brachytherapy and stereotactic body radiation therapy to deliver therapeutic radiation.
References:
- Hammers H, Plimack, ER, Infante JR, Ernstoff MS, Rini B, McDermott DF, Razak A, Pal SK, Voss MH, Sharma P, Kollmannsberger C, Heng D, Spratlin J, Shen Y, Kurland JF, Gagnier P, Amin A. Phase I study of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma (mRCC). ASCO 2014, Abstract #4504
- Hammers H, Plimack, ER, Infante JR, Ernstoff MS, Rini B, McDermott DF, Razak A, Pal SK, Voss MH, Sharma P, Kollmannsberger C, Heng D, Spratlin J, Shen Y, Kurland JF, Gagnier P, Amin A. Expanded Cohort Results From CheckMate 016: A Phase I Study of Nivolumab in Combination With Ipilimumab in Metastatic Renal Cell Carcinoma (mRCC). ASCO 2015, Abstract #4516
- Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM, Herati RS, Mansfield KD, Patsch D, Amaravadi RK, Schuchter LM, Ishwaran H, Mick R, Pryma DA, Xu X, Feldman MD, Gangadhar TC, Hahn SM, Wherry EJ, Vonderheide RH, Minn AJ. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015 Apr 16;520(7547):373-7.
- Wersäll PJ, Blomgren H, Pisa P, Lax I, Kälkner KM, Svedman C. Regression of non-irradiated metastases after extracranial stereotactic radiotherapy in metastatic renal cell carcinoma. Acta Oncol. 2006;45(4):493-7.