The JAVELIN Renal 101 Trial
Key points from the trial:
- This trial combined avelumab (immunotherapy) with axitinib (Inlyta – targeted therapy). Patients in the trial received either the combination treatment or sunitinib (Sutent).
- The primary goal of the study was to see if the combination therapy would increase Progression Free Survival (PFS) over what many patients receive as the standard of care (Sutent).
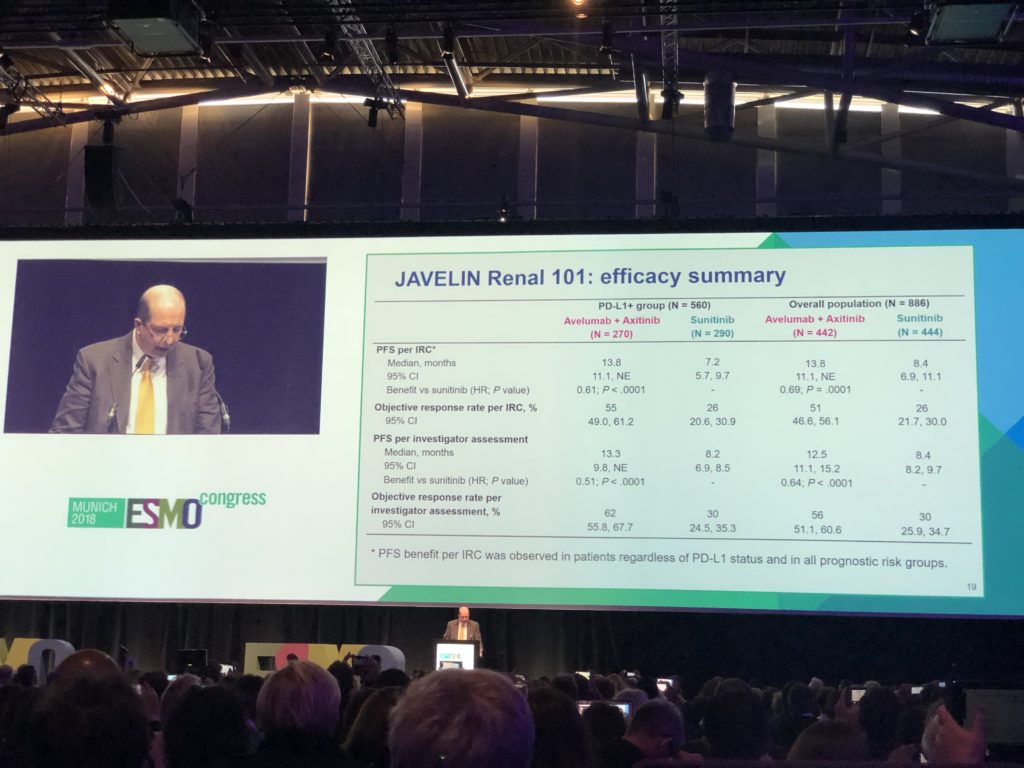
- The trial found that patients getting the combination therapy had progression free disease (PFS) for 13.8 months versus 8.4 months on sunitinib.
- Results also showed that 51 percent of patients on the combination therapy had an Objective Response (meaning their disease burden was reduced) versus 26 percent of patients on sunitinib.
What some experts are saying:
- Even though the trial is positive, one of the other endpoints of the trial was overall survival (OS). As patients live longer with kidney cancer, this measure can take longer to determine because patients have to be followed for a longer time. In this trial, it’s too early to tell the OS difference between the combination arm and the sunitinib arm.
- Because other combination therapies are also being tested in kidney cancer right now, (such as pembrolizumab and axitinib), many experts want to see the OS data as a measure of this combination versus other combination treatments.
- Unlike other combination therapies we have seen recently, such as ipilimumab and nivolumab, this combination treatment worked well in all patients, regardless of risk status or whether their disease was positive for PDL1, so this could offer a new option for low risk patients who have disease that might benefit more from anti-angiogenic therapy (targeted therapy).
What this means for patients:
- This trial is positive and that’s good news for kidney cancer patients.
- It’s likely that the manufacturer will quickly take steps to get approval from the U.S. FDA for this combination therapy as a treatment for metastatic kidney cancer.
- We can’t say with complete accuracy how long approval might take, but if this moves in the same way that other trials have moved forward, avelumab plus axitinib could be available in the first half of 2019.
- New therapy options add to the arsenal of treatments we have available to fight this disease. But questions still remain about what treatments work best for which patients and more research is needed!
To view more slides from the JAVELIN Renal 101 trial, visit our Facebook page!
Please note: The material on this site is for informational purposes only and is not a substitute for professional medical advice, diagnosis or treatment for a specific medical condition. You should not use information on this website to diagnose or treat a health problem or disease and should consult with a qualified healthcare provider with any questions or concerns you may have regarding your condition.